Updated on August 13, 2024.
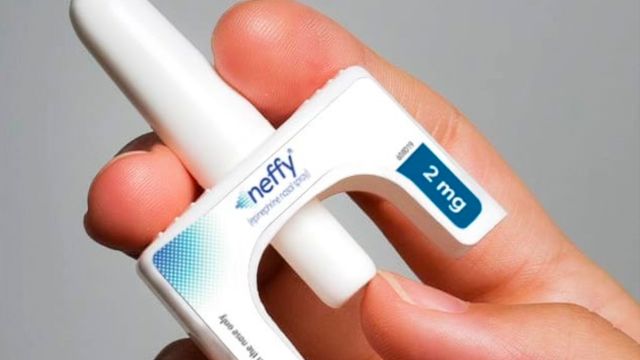
For the first time in nearly four decades, people with severe allergies have a new treatment option. On August 9, the U.S. Food and Drug Administration (FDA) approved neffy—a single-dose epinephrine nasal spray for the emergency treatment of allergic reactions, including anaphylaxis. The spray, which was developed by ARS Pharmaceuticals, is the first needle-free alternative to the EpiPen.
Neffy is approved for use in adults as well as children who weigh at least 66 lbs. The FDA’s landmark approval is based on four studies, which showed that neffy produced blood concentrations of epinephrine that were comparable to existing epinephrine injection products. The nasal spray also resulted in similar increases in blood pressure and heart rate—two effects that are critical in the management of life-threatening allergic reactions. A study involving children also showed that neffy is as effective among kids.
One prescription will include two nasal spray devices. These are the same spray devices used for the overdose reversal medication Narcan.
Each device contains a 2-milligram dose of epinephrine. It also includes an FDA-approved absorption enhancer. This allows the medication to be easily absorbed through membranes in the nose. The first dose is given in one nostril. If there is no improvement within 5 minutes or if symptoms worsen, the second dose from the additional nasal spray device may be given in the same nostril.
Nasal spray vs auto-injector: which one is better?
The FDA approved epinephrine auto-injectors in 1987. Since then, they have remained the standard treatment for life-threatening allergic reactions.
Roughly 40 million people in the U.S. have severe allergies, which are most often triggered by food, medications, latex, insect stings, or venom. But many people still hesitate to carry and use epinephrine auto-injectors.
For some, the fear of needles or injecting a needle into themselves or someone else is a deterrent.
“Anaphylaxis is life-threatening and some people, particularly children, may delay or avoid treatment due to fear of injections,” said Kelly Stone, MD, PhD, Associate Director of the Division of Pulmonology, Allergy and Critical Care in the FDA’s Center for Drug Evaluation and Research. “The availability of epinephrine nasal spray may reduce barriers to rapid treatment of anaphylaxis. As a result, neffy provides an important treatment option and addresses an unmet need.”
Improper training may also prevent people from using epinephrine auto-injectors correctly and with confidence. The most common mistake is not holding the pen against the thigh for at least a count to 10 to ensure delivery of the full dose of epinephrine.
Another reason why epinephrine may not be given as quickly as it should: Symptoms of anaphylaxis can be either unpredictable or develop gradually.
What is anaphylaxis—and could you spot it?
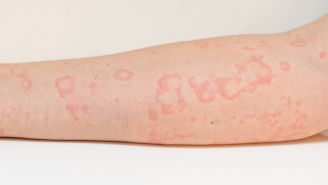
Anaphylaxis is a very serious allergic reaction. It can be deadly. It happens when the immune system overreacts to an allergen, such as a certain food or insect bite. It releases chemicals that trigger allergy symptoms.
Allergic reactions can be mild and just affect one part of the body, such as a skin rash or itchy eyes. But some people have more severe allergies. This can lead to anaphylaxis, which affects more than one part of the body at the same time. Warning signs may include:
- Red rash with hives that is usually itchy
- Swollen throat or swollen areas of the body
- Wheezing
- Passing out
- Chest tightness
- Trouble breathing, cough
- Hoarse voice
- Trouble swallowing
- Vomiting
- Diarrhea
- Stomach cramping
- Pale or red color on the face and body
- Sudden redness of the ears
- Feeling of impending doom
These symptoms usually start within 5 minutes of exposure to an allergen. But they can take up to 30 minutes to develop. This is why some people may not recognize that they are having a serious allergic reaction.
Anaphylaxis is a medical emergency. It must be treated immediately. If treatment is delayed or anaphylaxis is not treated, it can be fatal.
If you think that you or someone else is having an anaphylactic reaction, give epinephrine right away and call 911. Anaphylaxis should be evaluated and monitored by healthcare professionals for a period of time—even after epinephrine is administered.
What exactly is epinephrine?
Epinephrine is also known as adrenaline. It is a naturally occurring hormone—and a medication. It is the main treatment for anaphylaxis.
Epinephrine affects many different parts of the body, including the:
- Heart: It makes the heart pump faster and harder. This raises blood pressure and improves circulation.
- Lungs and airways: It makes people breath more deeply and faster. It dilates the airways and may reduce swelling.
- Eyes: It dilates the pupils.
- Skin: The skin may appear pale as blood is diverted to major organs and muscles.
- Muscles: It improves blood flow to the muscles.
Due to these effects, some people treated with epinephrine feel hyper or anxious.
What happens now?
Neffy is expected to be available within the next eight weeks. Its shelf life is 30 months. The spray can also withstand temperatures of up to 122°F. This means people could potentially store doses in their car. If neffy is accidentally frozen, it can also be thawed and used.
How much will it cost?
For those with private health insurance, neffy will come with a price tag of $25 per prescription through a co-pay savings program. One prescription includes two one-dose devices. ARS is also offering neffy for $199 via GoodRx or BlinkRX. Those who are underinsured or uninsured and meet eligibility criteria may be able to receive neffy for free through the ARS Pharma Patient Assistance Program (PAP).
What about side effects?
All medications come with risks for side effects. The most common side effects of neffy include:
- Throat irritation
- Tingling in the nose and elsewhere
- Headache
- Nasal discomfort
- Jitteriness
- Fatigue
- Tremor
- Runny nose
- Itchy nose
- Sneezing
- Belly pain
- Gum pain
- Numbness in the mouth
- Nasal congestion
- Dizziness
- Nausea or vomiting
People with certain conditions, such as nasal polyps or a history of nasal surgery, may not absorb neffy as well.
And those with certain medical conditions may be at greater risk for negative side effects from treatment with neffy or other epinephrine delivery devices, including those with heart disease or irregular heartbeats. People taking medications for certain conditions, including allergies, depression, thyroid disorders, diabetes, and high blood pressure, may also be at higher risk.
Neffy could also worsen symptoms of some conditions, including hyperthyroidism, Parkinson’s disease, diabetes, and kidney disease.
These people should talk to their healthcare provider (HCP) about the best treatment options for them.
Other options are in development
Neffy isn’t the only possible alternative to epinephrine auto-injectors. An under-the-tongue strip containing epinephrine is in clinical trials. The strip called Anaphylm is the size of a postage stamp. It immediately dissolves when placed under the tongue. The strip, which is made by Aquestive Therapeutics, has been shown to produce blood levels of epinephrine similar to auto-injectors.