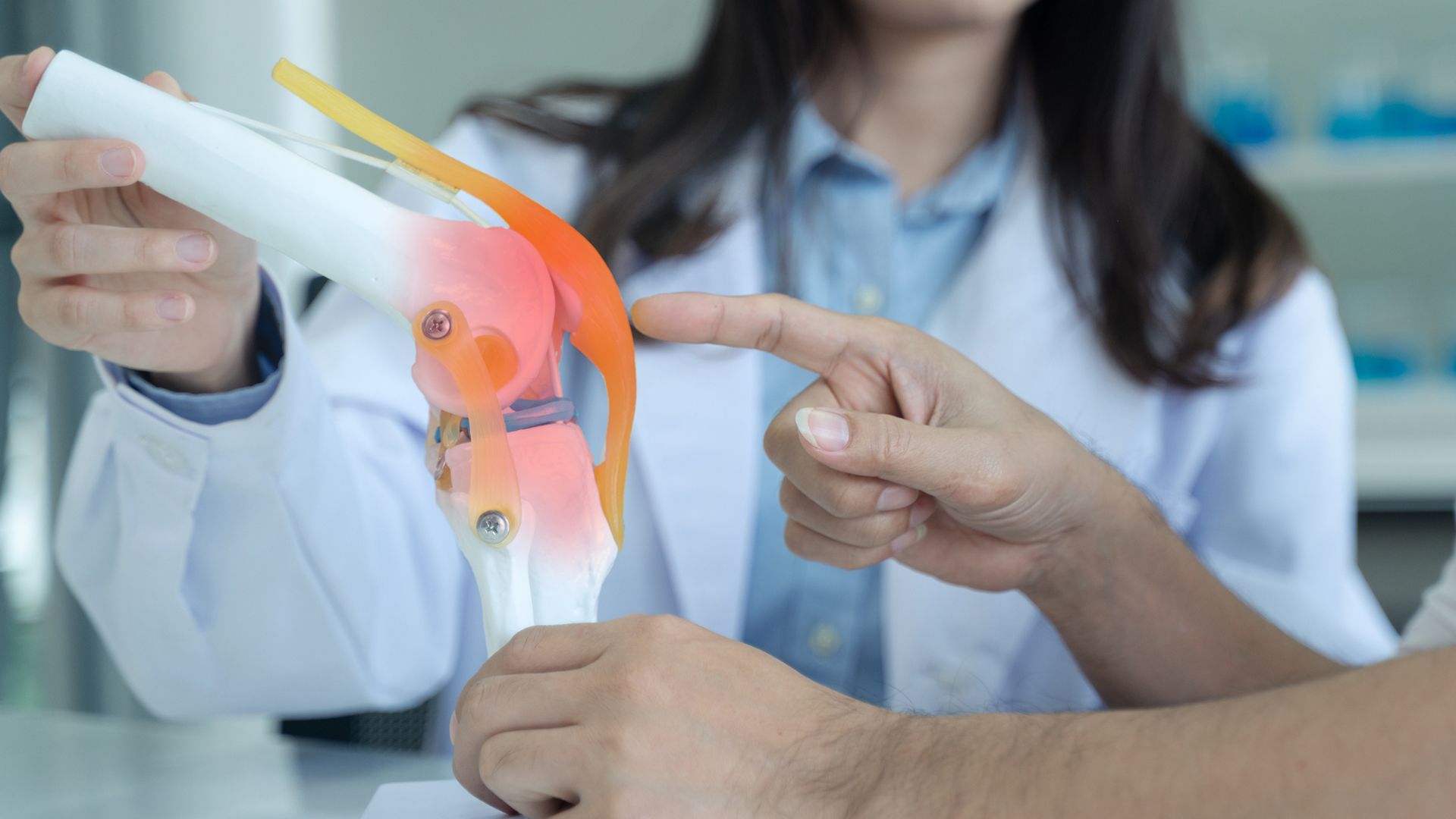
Surgery is the standard treatment for most cases of tenosynovial giant cell tumors (TGCTs), a rare type of benign tumor that form on the synovial membrane, a tissue that lines the joints. TGCTs can cause inflammation and pain, and in some cases can lead to disability. They can affect any joint throughout the body, ranging from small joints like the hands and feet, to larger joints like the knees, hips, and shoulders.
The surgical procedure most often used to treat TGCTs is called a synovectomy, and involves removing some or all of the synovial tissue in an affected joint. Surgery is sometimes used in combination with radiation therapy—either external beam radiation or radiosynovectomy—which can help shrink or destroy tumors and diseased tissues in the joints.
Though surgery is effective, TGCTs have a high rate of recurrence—for many patients, TGCTs come back after surgery. Some patients with this rare disease have undergone multiple rounds of surgeries to treat the condition, and some have undergone amputations and joint replacements as a result of TGCTs or complications from surgeries.
There are also some patients with TGCTs that are not able to have surgery, due to medical history or other health conditions.
It is for these reasons and others that healthcare researchers have actively investigated other potential treatments for TGCTs. In August 2019, the Food and Drug Administration approved an oral medication for the treatment for TGCTs. This medication is a kinase inhibitor.
Kinases are enzymes found in human cells. There are many different types. Different kinases signal different molecules within cells to become active or inactive. This helps control different cells functions, such as cellular division.
Kinase inhibitors are drugs that block kinases and stop these enzymes from working. Some kinase inhibitors are more selective than others—some block multiple types of kinases, while some block very specific types of kinases.
The kinase inhibitor indicated for the treatment of TGCT is a selective tyrosine kinase inhibitor—it blocks a kinase called colony stimulating factor 1 receptor (CSF1R). In tissues affected by TGCT, CSF1R is overexpressed, meaning that there is too much of it and it is too active, which contributes to the growth of TGCTs. Blocking CSF1R prevents the growth of TGCTs.
However, this therapy is not for everyone, and the decision to use this therapy is one that must be carefully considered by a patient and healthcare provider. The approval comes with a black box warning about severe liver problems, and the FDA requires all patients taking this therapy to participate in a Risk Evaluation and Mitigation Strategy (REMS) program, a safety program that aims to ensure the benefits of certain therapies outweigh the risks. REMS programs require special certification for healthcare providers and pharmacies, and there is an enrollment process for patients. This therapy is not advised for patients who have liver disease or raised liver enzymes, and patients taking this therapy must undergo routine liver monitoring. It is not advised for women who are pregnant, breastfeeding, or planning on becoming pregnant.
Always take a medication as directed by your healthcare provider, tell your healthcare provider about all medications that you are taking (including supplements, vitamins, and over-the-counter medications), and tell your healthcare provider about any side effects that you experience.