Updated on March 3, 2025
Meningococcal disease is a term for any illness caused by the bacteria N meningitidis. One of these illnesses is a bloodstream infection, which can cause skin and organs to bleed. Another is bacterial meningitis.
Bacterial meningitis happens when bacteria grow in the membranes around the brain and spinal cord, which are called meninges. It most often occurs in babies younger than 1 year old and in teens and young adults between the ages of 16 and 21. The symptoms come on quickly, and medical treatment with antibiotics is needed right away for the best chance of recovery.
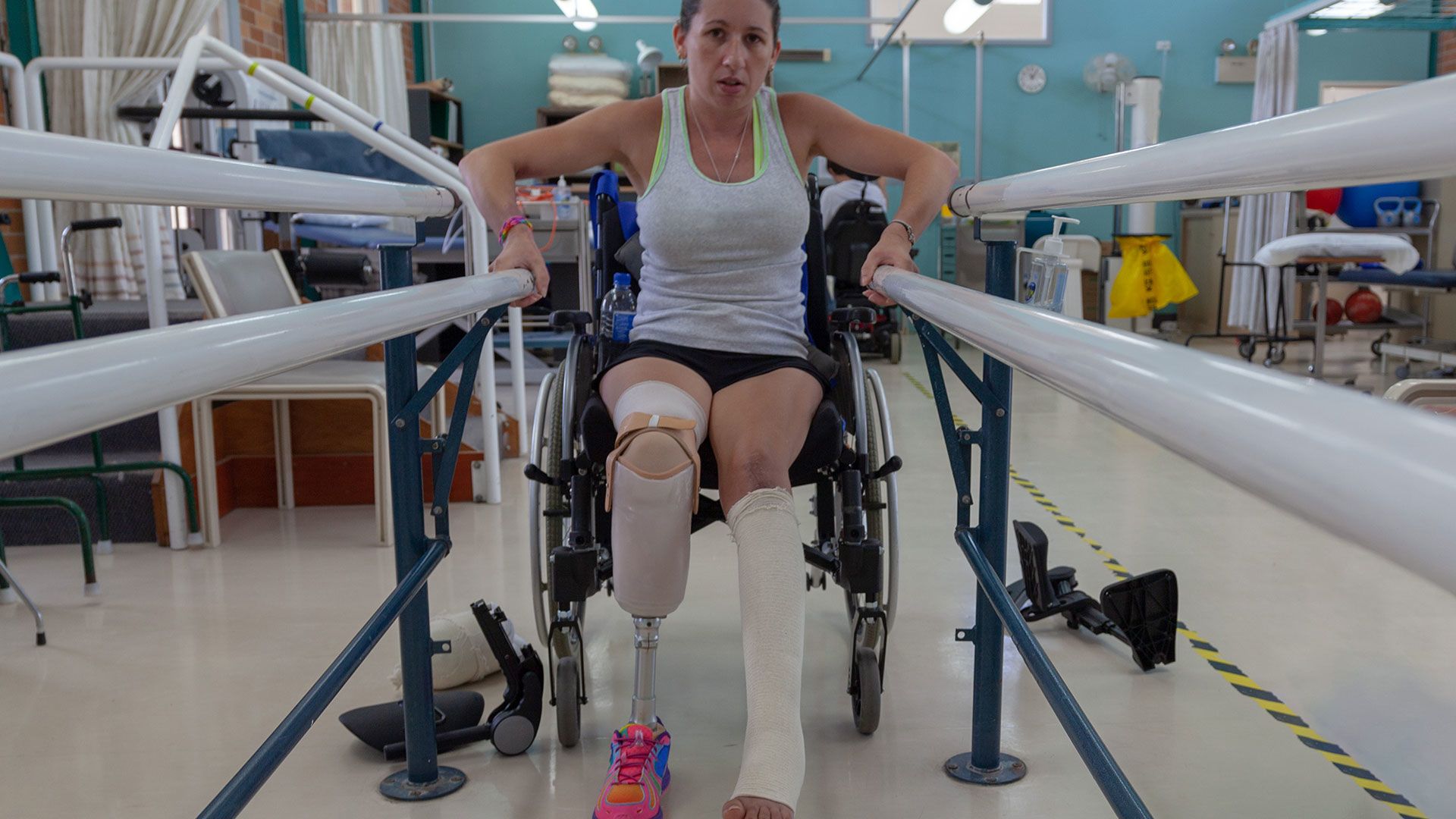
While bacterial meningitis is rare, it is life-threatening and typically devastating. Without treatment, many cases lead to death. Those who do survive may be left with long-term aftereffects: brain damage, paralysis, hearing loss, or the loss of an arm or leg.
When bacterial meningitis is treated early, outcomes tend to be much better. Death and permanent damage may still occur even with treatment, especially if there are delays. Up to 18 percent of people with bacterial meningitis die, even with treatment, according to the Centers for Disease Control and Prevention (CDC).
It’s best to avoid bacterial meningitis in the first place. There are vaccines, called meningococcal vaccines, that can help.
Types of meningococcal vaccines
There are many types, or serogroups, of the N meningitidis bacteria. Five common serogroups in the United States are A, B, C, W, and Y. Different vaccines help protect against different serogroups.
MenACWY: The vaccine nearly all adolescents get
The MenACWY vaccine protect against serogroups A, C, W, and Y. The CDC recommends MenACWY vaccination for all preteens and teens. The MenACWY vaccine is typically given at ages 11 to 12, with a booster dose given at age 16. It’s also recommended in certain high-risk adults and children 2 months and older.
MenB: The vaccine some people may get
The MenB vaccine may help protect against serogroup B. The CDC recommends that patients and healthcare providers decide together whether to get it.
The preferred timing for the vaccine is between ages 16 and 18, particularly if heading to a college dorm to live. Between 2013 and 2018, 10 outbreaks of meningococcal disease caused by the B serogroup occurred at colleges and universities in seven different states. As a result, many colleges and universities began to recommend or require that students get the MenB vaccine.
For most healthy teens, it is optional, though the CDC recommends it for some high-risk groups, as well as those at risk due to a local outbreak.
MenABCWY: The combined vaccine option some people may get
The MenABCWY vaccine helps protect against serogroups A, B, C, W, and Y. This combined vaccine is an option for adolescents and young adults ages 16 to 23 who would like to get MenACWY and MenB vaccines at the same visit.
Why isn’t the MenB vaccine recommended for everyone?
Given the potentially devastating effects of meningococcal disease, a person may wonder why MenB vaccination is not more widely recommended. The answer to this question has a lot to do with the need for more information before health experts can recommend routine MenB vaccination.
Meningococcal disease is rare in the United States. There are typically fewer than 200 each year caused by serogroup B, according to the American Academy of Family Physicians. Many of those cases occur in children younger than age 5 (an age group with no approved routine MenB vaccine). Many other cases occur among people who are 11 to 24 years old.
Health experts are always working to design tools and provide recommendations that could help people avoid serious illness. Many factors go into a vaccine recommendation. While blood tests in studies show that the MenB vaccine boosts immunity in people, evaluating how many infections the vaccine will prevent in real-world settings takes many years of data. The low number of cases makes it harder to confirm how effective getting the extra MenB vaccine is overall. It’s also unclear how long the immunity lasts after the series of shots.
Without this information, it is difficult for organizations like the CDC and the American Academy of Family Physicians to make routine recommendations for the entire population.
Public health experts need to also balance how effective something is in the real world with the vaccine’s possible side effects in patients. (Reported side effects listed by the CDC are mostly mild and last a few days, but serious reactions are possible.)
For these reasons, the MenB vaccine is optional for most young people today. In the meantime, the public health community continues to monitor all these factors to see if changes to the recommendations are needed.
When teens, young adults, and their parents decide to get a MenB vaccination, they are considering a completely different set of factors. These include the physical, emotional, psychological, and financial burdens that could come with meningococcal disease, including a bacterial meningitis infection that could result in brain damage, loss of limbs, or death.